Over the last year, several drugs have either been developed or tested to treat coronavirus. Now there’s another new antiviral drug that’s showing some promise by demonstrating ability to stop SARS-CoV-2 transmission within 24 hour!
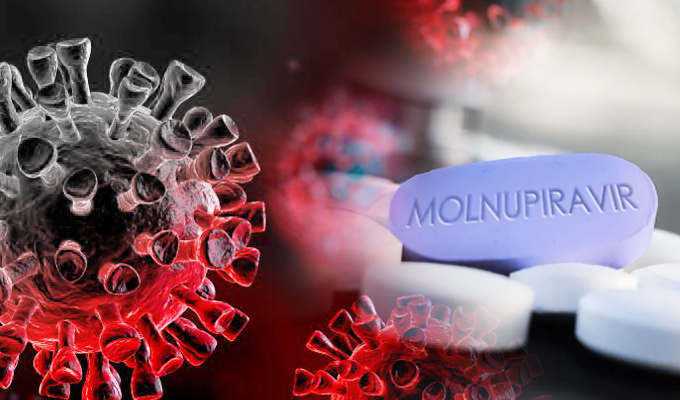
Molnupiravir an antiviral investigational drug administered orally is currently undergoing clinical trials.
Molnupiravir a ribonucleoside analog was first developed as preventative medicine in early 2000s has shown to work against many viruses that employ an RNA-dependent RNA polymerase, which SARS-CoV-2 also has. Molnupiravir doesn’t stop the virus from replicating, though; instead, the drug introduces errors into the virus’s RNA that are then replicated until it’s defunct.
In July 2020, Florida-based Ridgeback Biotherapeutics and US drug maker Merck & Co Inc. announced their partnership to advance development of Molnupiravir in Covid-19.
Merck is considering studying Molnupiravir as a preventive treatment, to be deployed after a person is exposed but before they’ve fallen ill. That would allow the drug to be deployed even more broadly in the fight against Covid. Should Merck succeed in demonstrating that Molnupiravir is effective and free of serious side effects, it could be a boon to the society, for many years to come.
During an animal study on ferrets, they tried to get coronavirus to spread, and it wouldn’t. So, while it’s a treatment designed to prevent hospitalizations and deaths; it also seems to prevent transmission.
With resources big enough for a trial, Merck launched Phase II/III trials with an aim to enroll almost 3,000 patients in the U.S., Colombia, Israel, Russia, and elsewhere. The company is checking constantly about the pace of recruitment of the study trial and hopes in sending safety efficacy data to the FDA to get emergency use authorization or even full authorization in record time.
ADVANTAGES
| It has been designed as an oral, unlike other treatments right now that are IVs which indicates it as a potential drug that could come before hospitalization and perhaps even prevent severe symptoms. |
| The drug can also be used for people who do not want to get a vaccine or don’t have the resources to get shots. |
| The drug is only used in the short term, twice-daily for five days. |
| It has shown promise against several RNA viruses, not just SARS-CoV-2, which would mean it could help governments prepare for the next pandemic. |
In India, decision to conduct human clinical trials with a new promising antiviral drug, Molnupiravir that is said to block covid transmission will be taken by the strategic group of scientist from Council of Scientific and Industrial Research (CSIR).
Dr. Shekhar Mande, Director General Council of Scientific and Industrial Research has suggested that “Like many other anti-flu drugs, Molnupiravir would also undergo clinical trials for testing in humans and they will apply to the drug regulator for approval.”
A previous study from India has also shown that whereas the SARS-CoV-2 virus can become resistant to Remdesivir, an injectable drug which showed promise in treating moderate Covid-19 disease, Molnupiravir may prove more effective in preventing drug resistance and emerge as a latest gamechanger for Covid-19 treatment.
“Even as the world cheers the progress being made on several vaccines to protect against the Covid-19 pandemic, there is great excitement now on Molnupiravir, a new anti-viral drug which according to latest research completely suppresses virus transmission within 24 hours.”
According to Mr. Yusuf Hamied Chairman of Cipla Limited, Merck has come to India and given all the generics companies an open license to produce this drug. Cipla is planning to do so and get approval in India.
Reference:
The floating traffic jams off ports. The multiplying costs of moving freight. The resulting shortages of goods. All of this had seemed like an unpleasant memory confined to the COVID-19 pandemic. But no such luck!
An ocean container capacity crunch has hit global trade just as peak shipping season starts, with freight spot rates up some 30% over the past few weeks and heading higher.
The first joint Europe-wide assessment of the drivers and impact of chemical pollution by the European Environment Agency (EEA) and the European Chemicals Agency (ECHA) has concluded that, despite progress in some areas, “more work is still needed to reduce the impact of harmful substances on human health and the environment”. Key findings include:
The severe drought which has forced the Panama Canal, one of the world’s busiest trade passages, to limit daily crossings could impact global supply chains during a period of high demand.
In the early hours of March 26, the Singapore-flagged ship Dali, loaded with 5,000 containers, slammed into Baltimore’s Francis Scott Key Bridge, causing the 1.6-mile (2.5-kilometer) bridge to collapse in a matter of seconds. The Dali was departing for Colombo when the disaster struck. Initial fears were confirmed that half a dozen people lost their lives in the accident.
The pharmaceutical and biotechnology industries constantly seek innovative methods to enhance product stability, solubility, bioavailability and ease of use. Within this realm, CDMOs [Contract Development & Manufacturing Organizations] serve as invaluable partners in the development and production of high-quality drug products.
Chinese New Year 2024 is upon us, disrupting logistics from Asia starting Feb 10th. This event is expected to impact global shipping until Feb 21. Freight rates from Asia has skyrocketed with rates to the US surging by 3.5X and Europe by 6X.
Amid ongoing Red Sea diversions by shipping giants like Maersk, CMA, logistics managers are globally confronting a dual challenge of escalating ocean and air freight prices alongside cargo disruptions due to
Why will CM be the next generation on quality?