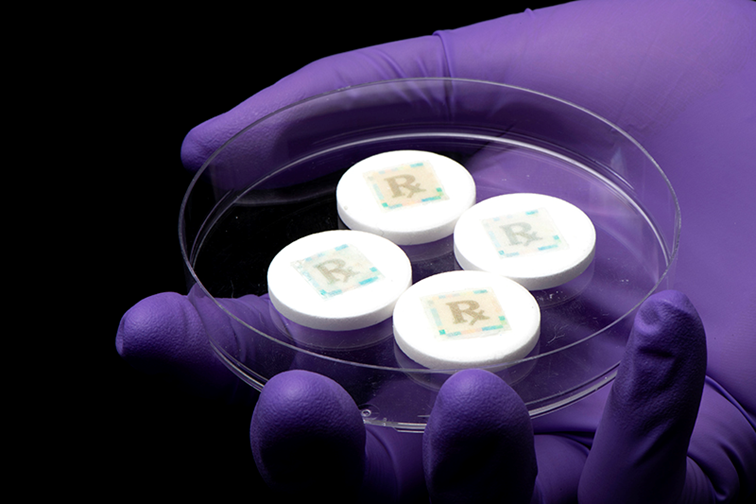
New anti-counterfeit technology, called a cyber-physical watermark, leverages edible fluorescent silk to identify medications.
The US Food and Drug Administration (FDA) has mandated that by 2023 medications have unit-level traceability through the Drug Supply Chain Security Act.
But how can this be achieved?
In a recent paper, researchers from Purdue University, US, revealed a technology that could meet this requirement.
Counterfeit medications and pharmaceutical products are just a click away from being purchased from online pharmacies via smartphone. However, the newly developed anti-counterfeiting technology could turn smartphones into lifesavers by allowing patients to simply take a picture of a cyber-physical watermark to confirm if a medication is real or not.
Purdue University biomedical engineers have developed new cyber-physical watermarks that allow people to check medication authenticity with a smartphone.
Young Kim, associate head for research and an associate professor in Purdue’s Weldon School of Biomedical Engineering, says the continued rise of counterfeit medications, pharmaceutical products and medical supplies can be attributed to the increase of online pharmacies, many of which are unregulated.
To counteract the issue, the FDA is requiring medications have unit-level traceability by 2023. But, while pharmaceutical companies currently have the ability to track boxes or sheets of medications, the jump to adding traceability directly to pills could require adding numerous manufacturing and data management steps, according to Kim.
Kim’s team proposes using small cyber-physical watermarks to trace medications and confirm whether they are real or fake, as well as allowing patients to confirm dose and frequency, and access additional information on the medicine.
People can use a smartphone app to activate a new cyberphysical watermark to detect whether the medication they are taking is real or fake. Purdue biomedical engineers have developed the cyberphysical watermark as a way to reduce fake medications.

“A paper watermark is commonly used on currency and a passport to discourage counterfeiting, and we are affixing a watermark on an individual medicine that is readable by a smartphone camera to extract a hidden digital key,” Kim said. “Purdue has an excellent track record of watermarking and inkjet printing research. We are proud that we have extended such national security research into pharmaceuticals as counterfeit medicines are a national security problem.”
The cyber-physical watermark is printed on specialised edible fluorescent silk with FDA-approved food dye using an inkjet printer. According to the researchers, using fluorescent silk is not only beneficial because it makes it difficult for counterfeiters to duplicate the watermark, but also because engineers can change the biopolymer’s shape, structure and flexibility. The engineers also addressed how to use the technology with different smartphone models, photo quality and light.
“A person can take a photo under different light conditions and will have different images. It is the same issue when patients take photos of our watermark in their phone,” Kim explained. “The reference colours on the watermark’s periphery allow us to know the true colour value of the watermarked image as each smartphone has different spectral sensitivity.”
Placed on pills using a simple sugar glue, the smallest size of watermark the team could produce is 5mm by 5mm. While the team has had success with solid pills, it is working also on developing technology for liquids.
Cyber-physical watermark technology could be used first on name-brand medications and restricted narcotics before being rolled out on over-the-counter medications and generics.
The floating traffic jams off ports. The multiplying costs of moving freight. The resulting shortages of goods. All of this had seemed like an unpleasant memory confined to the COVID-19 pandemic. But no such luck!
An ocean container capacity crunch has hit global trade just as peak shipping season starts, with freight spot rates up some 30% over the past few weeks and heading higher.
The first joint Europe-wide assessment of the drivers and impact of chemical pollution by the European Environment Agency (EEA) and the European Chemicals Agency (ECHA) has concluded that, despite progress in some areas, “more work is still needed to reduce the impact of harmful substances on human health and the environment”. Key findings include:
The severe drought which has forced the Panama Canal, one of the world’s busiest trade passages, to limit daily crossings could impact global supply chains during a period of high demand.
In the early hours of March 26, the Singapore-flagged ship Dali, loaded with 5,000 containers, slammed into Baltimore’s Francis Scott Key Bridge, causing the 1.6-mile (2.5-kilometer) bridge to collapse in a matter of seconds. The Dali was departing for Colombo when the disaster struck. Initial fears were confirmed that half a dozen people lost their lives in the accident.
The pharmaceutical and biotechnology industries constantly seek innovative methods to enhance product stability, solubility, bioavailability and ease of use. Within this realm, CDMOs [Contract Development & Manufacturing Organizations] serve as invaluable partners in the development and production of high-quality drug products.
Chinese New Year 2024 is upon us, disrupting logistics from Asia starting Feb 10th. This event is expected to impact global shipping until Feb 21. Freight rates from Asia has skyrocketed with rates to the US surging by 3.5X and Europe by 6X.
Amid ongoing Red Sea diversions by shipping giants like Maersk, CMA, logistics managers are globally confronting a dual challenge of escalating ocean and air freight prices alongside cargo disruptions due to
Why will CM be the next generation on quality?