INTRODUCTION
Ammonium acetate is a crystalline white solid with a somewhat acetous odour. It is also called the spirit of Mindererus in its aqueous form or Azanium acetate. It is widely used in chemical analysis, in preserving foods, and in pharmaceuticals. It is often used to prepare buffer solutions as it is made up of a weak base and a weak acid combined together.
Manufacture
Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with ammonia.
| Synonyms | Acetic acid amine Ammonium ethanoate |
| CAS no. | 631-61-8 |
| EINECS no. | 211-162-9 |
| Molecular formula | C2H7NO2 |
| Molecular weight | 77.08 |
| Structure | 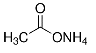 |
Applications
| Ammonium acetate is commonly used as a controlling agent for food acidity. It alters or regulates the alkalinity or acidity of foods. |
| It is also commonly employed in the Knoevenagel condensation technique as a catalyst. The Borch reaction during organic synthesis uses the molecule as one of the greatest sources of ammonia. |
| A protein precipitating reagent is made by combining ammonium acetate with completely distilled water. |
| The substance may also be used as a buffer in ESI (electrospray ionization) mass spectrometry of molecules and proteins, as well as a mobile phase in HPLC (high-performance liquid chromatography). |
| It is used in the production of explosives. |
| It is used for making Foam rubber & Vinyl plastics. |
| In analytical chemistry, the compound is used in the form of a reagent. It is used as a reagent in different dialysis procedures for the elimination of contaminants through diffusion. |
| In agricultural chemistry, ammonium acetate, when used as a reagent, helps in determining soil CEC or cation exchange capacity along with the availability of potassium in the soil. |
SPECIFICATIONS
| Test | Unit | Specification |
|---|---|---|
| Description | – | Colorless hygroscopic crystals |
| Assay | % | Min. 96.0 |
| pH (5% solution) | – | 6.5 – 7.5 |
| Chloride (Cl) | % | Max. 0.005 |
| Sulphate (SO4) | % | Max. 0.01 |
| Iron (Fe) | % | Max. 0.001 |
| Heavy Metals (as Pb) | % | Max. 0.001 |
STORAGE
Store in cool dry place.
PACKING
25 kg bags.
REACH status
Ammonium acetate offered by ExSyn is EU REACH registered.
No matter the quantity you need, our exceptional quality and service will make ExSyn your supplier of choice! If you need any additional information or SDS, please contact us.
1,4,7-Trimethyl-1,4,7-triazonane (Me₃TACN) is a valuable macrocyclic tridentate ligand derived from 1,4,7-triazacyclononane. In Me₃TACN, each nitrogen atom is substituted with a methyl group, which significantly modifies its steric and electronic properties. Me₃TACN is widely used as a ligand in coordination chemistry due to its ability to form stable complexes with transition metals such as Cu, Ni, Fe, Mn, and Zn.
Adenosine is a naturally occurring purine nucleoside composed of adenine and ribose, present in all living cells. It plays a vital role in biochemical processes such as energy transfer (ATP/ADP), signal transduction, and regulation of physiological functions, and is widely used in pharmaceutical applications.
Methyl-2-Methoxy-5-Sulfamoyl Benzoate (MSMB) is a sulfur containing organic building block acting as a chemical intermediate. MSMB contains ester and sulfamoyl functional groups, which make it useful as a pharmaceutical and fine-chemical intermediate.
Imidazolidinyl urea is a widely used antimicrobial preservative in the cosmetic and personal care industry. It is a formaldehyde-releasing compound that effectively inhibits the growth of bacteria, yeast, and molds, thereby extending the shelf life of formulations. Due to its broad-spectrum efficacy and cost-effectiveness, it is commonly used in rinse-off and leave-on products within regulated concentration limits.
Phosphorodiamidites are a unique class of phosphorus-based compounds characterized by one P–O bond and two P–N moieties. 2-Cyanoethyl tetraisopropylphosphorodiamidite plays a crucial role in solid-phase oligonucleotide synthesis. Beyond their application as synthetic precursors for oligonucleotides, phosphorodiamidites have also been reported as valuable starting materials for the synthesis of industrially relevant polymers and flame-resistant materials, including adhesives, coatings, and laminates.
Ferric maltol is an oral iron complex composed of ferric iron (Fe³⁺) chelated with maltol, designed to improve iron absorption while reducing common gastrointestinal side effects associated with traditional iron salts. It is primarily used in the management of iron deficiency with better tolerability.
Phosphatidylcholine (PC) is one of the most important phospholipids derived from soya lecithin and represents a key structural component of cell membranes. Phosphatidylcholine 90% refers to a highly purified grade where the PC content is enriched to around 90% through specialized extraction and purification steps.
Indorez® Kota Resin is a modified non self-cured phenolic resin, it can increase rubber adhesion to steel, polyester, rayon, nylon, aramid and other fabric cord. It’s an adhesive agent, acts as methylene acceptor, can 100% replace resorcinol and resorcinol resins in resorcinol- formaldehyde-silica, enhance adhesion between rubber and reinforcing materials.
Glyceryl Stearate Citrate (GSC) is a plant-derived, biodegradable emulsifier commonly used in cosmetics and personal-care products. It functions primarily as an anionic oil-in-water (O/W) emulsifier, helping blend oils and water into smooth, stable creams and lotions. Its natural origin and gentle profile make it popular in eco-certified, organic, and sensitive-skin formulations.
