INTRODUCTION
Trifluoroacetic acid (TFA), is widely used in organic synthesis as a solvent, catalyst and reagent thanks to its reactivity and stability under adverse conditions.
It is considered one of the most economical fluorinated building blocks. It is widely used in peptide synthesis and other organic transformations involving deprotection of t-BOC group.
Manufacture
Trifluoroacetic acid is commonly produced in Simon electro-fluorination process involving hydrogen fluoride and acetic acid.
| Synonyms | Trifluoroethanoic acid Perfluoroacetic acid |
| CAS no. | 76-05-1 |
| EINECS no. | 200-929-3 |
| Molecular formula | C2HF3O2 |
| Molecular weight | 114.02 |
| Structure | 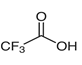 |
General Usage
The acid owes its multiple usages to its low boiling point, high dielectric constant, and relatively stronger acidity. It is generally employed in chemical industry as:
| Solvent in polymerization and oxidation |
| Solvent for chromatography |
| Catalyst in esterification, transesterification, and olefin reduction in petroleum refining |
| Low Boiling Reagent in various reactions |
Niche’ Applications
The chemical processes, that lead to value added products, are centered around the carboxylic group of trifluoroacetic acid and the stability rendered by the trifluoromethyl group. The special applications include:
| Trifluoromethylation – introduction of CF3 group in organic molecules thereby creating CF3 building blocks |
| Hydroacylation of complex organic molecules |
| Acylation of aromatics to form corresponding ketones and a plethora of acyl-containing intermediates |
| Olefin Hydration to produce alcohols – it replaces sulphuric acid, to which olefins are sensitive |
| Deprotection of amino group in some peptide synthesis for its higher solvency and volatility |
| Rearrangement – the acid is a key reagent in converting cyclohexanone oxime to ε-caprolactam |
| Protein Synthesis – where the acid protects the active amine |
Proxime Intermediates
The fluorinated acetic acid, by conventional synthetic processes, is easily converted to next step intermediates, that are vital for pharma and agro industries.
In these processes as well, the active carboxylic group and the stable trifluoromethyl moiety are the factors responsible.
These include:
| Trifluoroacetic anhydride – that further produces Thiazafluron |
| Trifluoroacetic acid ethyl ester (also known as ethyl trifluoroacetate) |
| Trifluoroacetic acid methyl ester |
| Trifluoroacetic acid isopropyl ester |
| 2,2,2-Trifluoroethanol – that is in turn consumed to make isoflurane and polythiazide |
High-End Intermediates
Trifluoroacetic acid finds applications as a key raw material to manufacture number of value-added, high-end intermediates such as:
| Peroxytrifluoroacetic acid |
| 2-(2,2,2-Trifluoroacetylamino)pyridine |
| N[1-{6-Chloro-3-pyridinyl)methyl)-2(1H)-pyridinylidene]-2,2,2, trifluoroacetamide |
| Methyl 2-Fluoroacrylate |
SPECIFICATIONS
| Test | Unit | Specification |
|---|---|---|
| Appearance | – | Clear & colorless liquid |
| Moisture | % w/w | 0.05 max |
| Fluoride | % w/w | 0.005 max |
| Chloride | % w/w | 0.001 max |
| Sulphate | % w/w | 0.001 max |
| Arsenic | ppm | NMT 8.0 |
| Titrimetric Purity | % w/w | 99.9 min |
| Identification by Ion Chromatography | The retention time of TFA matches to that of the standard TFA |
REACH status
TFA offered by ExSyn is registered under EU REACH regulations.
STORAGE
Product is stored at ambient temperature.
ExSyn offers this product on commercial scale and welcomes enquiries. No matter the quantity you need, our exceptional quality and service will make ExSyn your supplier of choice! If you need any additional information or SDS, please get in touch with us.
Imidazolidinyl urea is a widely used antimicrobial preservative in the cosmetic and personal care industry. It is a formaldehyde-releasing compound that effectively inhibits the growth of bacteria, yeast, and molds, thereby extending the shelf life of formulations. Due to its broad-spectrum efficacy and cost-effectiveness, it is commonly used in rinse-off and leave-on products within regulated concentration limits.
Phosphorodiamidites are a unique class of phosphorus-based compounds characterized by one P–O bond and two P–N moieties. 2-Cyanoethyl tetraisopropylphosphorodiamidite plays a crucial role in solid-phase oligonucleotide synthesis. Beyond their application as synthetic precursors for oligonucleotides, phosphorodiamidites have also been reported as valuable starting materials for the synthesis of industrially relevant polymers and flame-resistant materials, including adhesives, coatings, and laminates.
Ferric maltol is an oral iron complex composed of ferric iron (Fe³⁺) chelated with maltol, designed to improve iron absorption while reducing common gastrointestinal side effects associated with traditional iron salts. It is primarily used in the management of iron deficiency with better tolerability.
Phosphatidylcholine (PC) is one of the most important phospholipids derived from soya lecithin and represents a key structural component of cell membranes. Phosphatidylcholine 90% refers to a highly purified grade where the PC content is enriched to around 90% through specialized extraction and purification steps.
Indorez® Kota Resin is a modified non self-cured phenolic resin, it can increase rubber adhesion to steel, polyester, rayon, nylon, aramid and other fabric cord. It’s an adhesive agent, acts as methylene acceptor, can 100% replace resorcinol and resorcinol resins in resorcinol- formaldehyde-silica, enhance adhesion between rubber and reinforcing materials.
Glyceryl Stearate Citrate (GSC) is a plant-derived, biodegradable emulsifier commonly used in cosmetics and personal-care products. It functions primarily as an anionic oil-in-water (O/W) emulsifier, helping blend oils and water into smooth, stable creams and lotions. Its natural origin and gentle profile make it popular in eco-certified, organic, and sensitive-skin formulations.
DL-Serine is a racemic mixture of both the D- and L-forms of the amino acid serine, which is a polar, nonessential amino acid. It is an α-amino acid characterized by its hydroxyl-containing side chain, which enables it to take part in numerous biochemical reactions and synthetic processes. It functions as a pivotal intermediate in the biosynthesis of amino acids, including glycine and cysteine, underscoring its essential role in cellular metabolism. Moreover, it constitutes a critical pharmaceutical intermediate for the synthesis of a broad spectrum of therapeutic agents and drug candidates.
Ethyl vinyl ether is a reactive, flammable, volatile liquid with a strong ether-like odor. Featuring two conjugated functional groups—an ether and an alkene—this molecule act as important building block, especially in polymer synthesis. Its applications span multiple industrial sectors, including semiconductors, coatings, inks, fragrances, adhesives, paints, oil viscosity modifiers and pharmaceuticals, with promising potential as a dietary supplement.
Peppermint (Mentha piperita) is a common herb, also known as a hybrid mint. Its main components are oxygenated monoterpenes: alcohols, esters and ketones. Peppermint oleoresin microencapsulated powder is a white to off-white coloured powder produced from the seeds of the plant. In order to protect and maintain the stability of peppermint oil, microencapsulation is carried out through process optimization using the coacervation technique. This technique helps limit the loss and degradation of flavours and aromas during processing and storage. It offers versatile applications across multiple industries — from food and beverages to pharmaceuticals, cosmetics, and textiles.